Federal Reporting
Learn more about federal reporting with Veradigm’s Cardiology and Metabolic Registry in Partnership with the American College of Cardiology Qualified Clinical Data Registry.

The following information is provided for educational purposes only and should not be regarded as clinical or legal advice. Veradigm does not ensure the accuracy of this information and does not guarantee that following this information will result in receiving any government payment. It is the attesting healthcare providers responsibility to comply with all program requirements.
Quality Payment Program (QPP)
The Veradigm Cardiology Registry and Metabolic Registry captures valuable data on coronary artery disease, hypertension, heart failure, atrial fibrillation and diabetes in the outpatient setting to provide clinical insights to practices. The registries have been approved as a single Qualified Clinical Data Registry (QCDR) for the 2026 Merit-based Incentive Payment System (MIPS) Program Year, offering eligible participating practices a free and easy solution to report MIPS data in 2026.
Practices must be currently submitting data via System Integration (SI) or Secure File Transfer Protocol (SFTP) to have the registries submit MIPS data to CMS on their behalf.
Benefits of reporting via the Veradigm Cardiology and Veradigm Metabolic QCDR:
- Reporting MIPS data to CMS is a FREE benefit to eligible practices submitting data through the registries
- Interoperability with most electronic health record (EHR) systems
- Flexibility to select your six Quality measures, Promoting Interoperability measures and Improvement Activities that are specific to your practice through an easy-to-use interactive MIPS Dashboard
- Practices have the option to report MIPS or MVP
- Practices have the option of reporting at the individual provider level or at the group level (>2 clinicians with at least one MIPS eligible provider, who have reassigned their Medicare billing rights to a single TIN)
- When a practice reports as a group, their data is scored by CMS at the practice level. Group reporting also means that performance data on Care Compare is posted at the group level, not individual provider level
- Frequent feedback and monitoring: Participants receive access to quality improvement tools and clinical support, as well as monthly benchmarking reports that include registry and CMS benchmarks for comparison purposes
Quality Measures
Measures indicated as available for public reporting on Care Compare are required to meet certain standards set by CMS. These measures must be statistically valid, reliable, accurate, comparable across submission mechanisms and meet the minimum reliability threshold to be included in the Provider Data Catalog. The measures are posted publicly in plain language, making them easier to understand. Measures in their first two years, measures with fewer than 20 reporters, non-risk adjusted QCDR outcome and non-proportional measures are not available for public reporting.
2026 Program Year
Details on the CMS approved 2026 QPP measures for the Veradigm Cardiology and Metabolic QCDR are below:
2026 Clinical Data Registry QPP Timeline
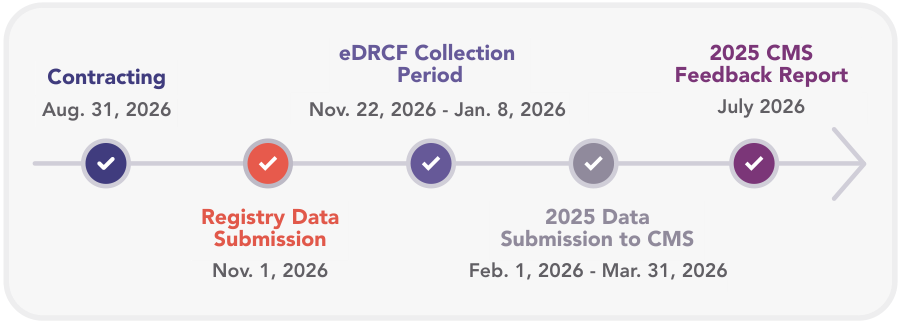
Requesting use of the QCDR for Reporting
Eligible providers who are actively submitting data to the Veradigm Cardiology and/ or the Metabolic Registry can request we report MIPS data on their behalf to CMS via the QCDR. To initiate this, clinicians must complete an electronic data release consent form (eDRCF). Groups reporting will only need to sign one eDRCF while individual providers are required to submit one eDRCF per reporting provider.
The eDRCF collects clinician and group consent. This is an annual requirement by CMS that allows the QCDR to release data to CMS on the clinicians’ or groups’ behalf. Only data for eligible providers and groups will be submitted to CMS if the QCDR receives a completed eDRCF by the specified deadline. Starting in 2025, all MIPS participants are required to submit their CMS 1500 form for tax identification number validations.
For more information, see our MIPS Frequently Asked Questions.
Additional Information
- 2025 QPP Final Rule Fact Sheet
- 2025 QPP Final Rule External FAQs
- 2025 MIPS Checklist
- 2025 MVP Checklist
- If you have questions about the Clinical Data Registries and participating in MIPS, contact the Clinical Data Registries Support Team at (833) 644-7466 or registries@veradigm.com
- If you have an inquiry regarding the reporting requirements, payment adjustments and performance feedback reports please contact CMS, available Monday - Friday 8:00 a.m. - 8:00 p.m. ET by phone 1-866-288-8912 (TRS:711) or email at QPP@cms.hhs.gov.
- For more information about MIPS and how to get started please visit https://qpp.cms.gov/
Already a Registry Participant?
Log In