Unveiling New Research on the Effects of Persistence vs. Discontinuation of GLP-1 Receptor Agonists – ISPOR Europe, 2024

Written by: Cheryl Reifsnyder, PhD and Janna Manjelievskaia, Director of Health Economics and Outcomes Research, Veradigm
Glucagon-like peptide 1 receptor agonists (GLP-1 RAs) have established efficacy in glycemic control and weight loss in adult populations. Emerging evidence suggests that GLP-1 RAs may have clinical implications beyond a weight loss and glycemic control indication, including cardiovascular disease, nonalcoholic fatty liver disease, and recurrent alcohol use disorder. In this article, we share some recent research utilizing Veradigm Network EHR data linked with claims data. This research compares the effects of GLP-1 RA medications indicated for weight loss on patients who persisted in medication usage versus those who discontinued use. Keep reading to learn what insights we’ve uncovered regarding the effects of 3 GLP-1 RA medications.
Study: Impact of GLP-1 RA persistence vs. discontinuation
Veradigm researchers used claims-linked Veradigm Network EHR data to assess the effects of 3 GLP-1 receptor agonists: semaglutide, liraglutide, and tirzepatide between 2021-2023. Assessments were based on changes in patients’ body mass index (BMI), total cholesterol, HDL-C, LDL-C, triglycerides, apolipoprotein B, lipoprotein(a), and hemoglobin A1c (HbA1c) over a 2-year follow-up period in patients who persisted in taking their medication versus those who discontinued use.
The study included 193,090 adult patients new to GLP-1 RA use. Included patients were required to have data available for ≥12 months prior to treatment initiation and ≥ 24 months after start of treatment. Patients’ mean (SD) age was 55.5 (11.3) years, and 59% of patients were female. The median BMI was 34.6. Nearly a third (28%) of patients were persistent on GLP-1 RA therapy over the 24-month follow-up period.
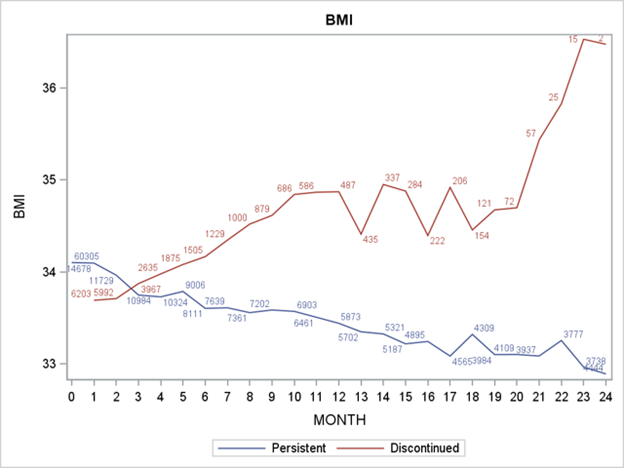
Figure 1: Impact of persistence vs discontinuation of GLP 1- RAs on BMI
Compared to baseline BMI, discontinuers had a similar BMI (34.2) during the 2-year follow-up period, whereas persistent patients saw a decrease in median BMI (33.6). Similarly, patients who discontinued did not see a change in median A1c from baseline to follow-up (7.1), whereas median A1c decreased among persistent patients (6.9). Similar trends were observed for cholesterol, and other lipid measures. Specifically, for total cholesterol, patients who discontinued saw a median value increase beyond their baseline measure following discontinuation.
Based on these data, we concluded that improvements in BMI, glucose control, and lipids were seen in patients persistent on GLP-1 RAs. However, following discontinuation of medication, patients’ clinical markers either returned to baseline levels or worsened—suggesting that patients need to remain on to maintain benefits.
Future work analyzing GLP-1 RAs will leverage Veradigm’s proprietary NLP enhanced EHR data to support expanded analysis.
ISPOR Europe, 2024
Are you attending the 2024 International Society for Pharmacoeconomics and Outcomes Research (ISPOR) Europe conference? We will present this research there in greater detail (ISPOR Europe, November 17-20, 2024). Connect with us in Barcelona to discuss—or contact us here to learn more: