4 Ways Real-World Data is Revolutionizing Cardiology Care

Written by: Nicole Darden, Product Manager, Veradigm
Cardiology is dedicated to diagnosing, treating, and managing cardiovascular diseases, where groundbreaking discoveries are made continually.
The availability of real-world data will advance the field of cardiology even further. By incorporating real-world data into everyday practices and clinical research, cardiology will grow in its capacity to make accurate diagnoses and effectively manage cardiovascular diseases.
Real-world data (RWD), defined by the FDA as “data relating to patient health status and/or the delivery of health care routinely collected from a variety of sources,” comes from a surprising number of sources. Electronic health records (EHRs) and registries are two prominent starting points for producing information. Veradigm Network EHR Data is one of the largest EHR data products designed exclusively for research, with more than 154 million deidentified patients with clinical activity and more than 246,000 providers.1
Registries are another strong data source, such as the Veradigm Cardiology Registry, which operates in partnership with the American College of Cardiology (Table 1).
Table 1. The Veradigm Cardiology Registry is the Largest Outpatient Registry for Coronary Artery Disease, Hypertension, Heart Failure, and Atrial Fibrillation.2
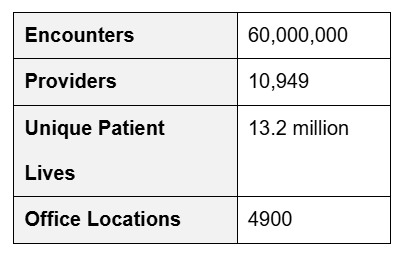
Hospital data and information gathered from personal health devices also contribute to the growing pool of real-world data, which already provides a wealth of continuous insights today.
Here are four ways real-world data is transforming the field of cardiology.
#1—Randomized Clinical Trials Will Have Larger Real-World Components
For years, randomized clinical trials (RCTs) have set the standard for trustworthy and dependable science. RCTs present some of the best science possible because they allow researchers to control study conditions, randomly assign treatment groups, use blinding or masking techniques to minimize bias and prescribe outcome measures.
The missing element from these carefully controlled conditions is how a therapy will act in the real world—a world that does not adhere to the rigid designs and limited timeframes of RCTs. For instance, how might a therapy interact with the unforeseen conditions of everyday life? Are there rare adverse events? How will the therapy work over an exceptionally long time? These are some questions that RCTs do not or cannot answer.
Real-World Data Supplements Randomized Clinical Trials
RWD may, at times, provide insights where RCT could not. Adding RWD when studying a rare cardiovascular condition, for instance, may yield more insight than an RCT could provide alone.
Electronic Health Records
EHRs were created precisely to record real-world symptoms, diagnoses, treatments, and treatment outcomes. The data from EHRs include routine clinical care in health clinics, hospitals, and other healthcare entities. EHRs can also capture environmental factors and social determinants of health information, such as food insecurity or access to transportation. EHR data comes from health clinics, hospitals, and other healthcare institutions.
Disease and Product Registries
Registries are organized databases for collecting and analyzing observational data on specific populations. Registries collect standardized, prospective data in real-world settings. Often, registries are narrowly focused and collect detailed information for a specific population over time.
Disease registries collect data from patients diagnosed with specific conditions or diseases. These tend to collect information about disease onset, symptoms, treatments, and outcomes.
Medical product or device registries are focused on patients who are using a particular medical product or device. These registries are usually created to support post-marketing surveillance. They often contain rich information about the product or device but more limited data on patient characteristics.3, 4
Administrative Claims
Administrative claims data include billing and other healthcare utilization data generated when healthcare claims are processed by health insurance plans or practice management systems. Claims data are collected and stored primarily for payment purposes.5 They include the services patients receive during each healthcare encounter, such as treatment and clinical data, diagnosis codes, and hospital admission/discharge information.6
Digital Health Technologies
Smart watches, mobile phones, and continuous glucose monitors are common digital health technologies, among many others. These digital tools generate vast amounts of data, which can be a problem for storage and analysis. The digital tools also may generate data that may not represent a sufficiently broad patient population.
Randomized Clinical Trials May Embrace Real-World Data
How will cardiologists use near real-time, standardized, real-world data as they conduct randomized clinical trials?
- Real-world data can aid in clinical trial recruitment: EHR and claims data can help identify larger numbers of patients who might be eligible for a cardiology trial. Registries and EHRs can help screen and identify patients who meet the inclusion criteria—making recruitment easier. AI (artificial intelligence) can translate complex clinical trial requirements and information into plain language that is more accessible to potential study participants.
- Real-world data provides comprehensive baseline characteristics: Large-scale demographics, medical histories, lifestyle factors, and other data can all be used to stratify patient groups in randomized clinical trials. The result could improve the statistical power of each study.
- Long-term outcomes and safety monitoring: Real-world data allows researchers to evaluate the outcomes of an intervention long after the randomized clinical trial has ended. This longitudinal data will yield insights into the intervention’s real-world durability and safety.
#2—Cardiology Patients Can Be Supported at Home
Personalized Remote Monitoring
Remote monitoring and other digital health technologies expand the opportunities for monitoring patients with chronic conditions, like heart failure, while the patient remains in their home. In their review of cardiology telemonitoring, Kinast, Lutz, and Schreiweis recognize the power of remote monitoring:
“This potential has been recognized by both researchers and health care professionals, as remote patient monitoring opens up new sustainable ways to support and care for patients in their homes.”7
Real-world data from mobile devices can track the key physiological signals that indicate a patient’s general condition, for instance, heart rate, blood pressure, and weight. Recognizing worsening symptoms is key to managing conditions like heart failure, which affects 26 million people worldwide. Remote monitoring can reduce hospital readmissions by helping providers intervene when needed.8
Predictive Analytics Show Risks of Adverse Events
Advanced analytics used on real-world data will identify patterns or biomarkers that predict the likelihood of adverse events, such as a heart attack or some heart failure complication. Real-world data helps to stratify patients or categorize them according to risk: low-, medium-, or high-risk. Given the risk level, tailored treatment plans can be offered to each patient.
#3—Cardiology Patients Can Self-Monitor for Health
Personal Health Data Collection
Regular measurement of cardiac vital signs is simplified through smartwatches, mobile phone apps, and for-purpose devices like continuous glucose monitors and blood pressure monitors. Each of these personal, real-world data sources will impact the cardiology patient in different ways (Table 2).
Table 2. Personal Health Trackers and Outcomes.
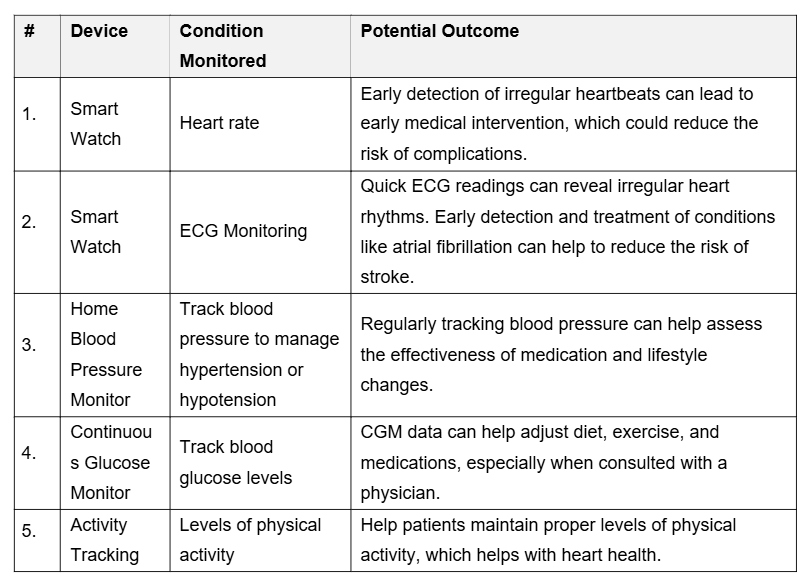
#4—RWD Can Help Cardiologists Understand Which Therapies Are More Effective
EHRs Provide Longitudinal Data
EHRs provide a comprehensive look at patient data, such as medical history, diagnoses, treatments, lab results, and imaging, which can demonstrate a particular therapy’s effectiveness (or lack thereof) over time. Veradigm Network EHR Data delivers approximately 2 years of retrospective longitudinal data. Data from electronic health records may also allow analysis across different patient demographics and comorbidities to fine-tune understanding of different patient groups.
Disease and Product Registries Point to Comparative Effectiveness
Registries are excellent at collecting standardizing data and aggregating results for insights about patient populations. Registries can help cardiologists by aggregating data from many patients treated with different therapies to compare the efficacy of the outcomes.
One example of gauging therapy delivery effectiveness was when researchers used the Veradigm Cardiology Registry (known as the PINNACLE Registry at the time) to assess over 855,000 patients from 400 practices receiving aspirin for primary prevention. Their analysis revealed inappropriate use of aspirin in 27.6% of patients evaluated and used without a recommended indication in 26.0%.”9
Administrative Claims Can Help Establish Cost-Effectiveness
Billing information and healthcare utilization data, which are part of administrative claims, can help cardiologists understand the economic impact of different therapies. Rates of hospital readmissions can also help determine a therapy’s effectiveness.
Digital Health Technologies Can Provide Behavioral Data
Wearables, mobile apps, and remote monitoring devices capture data continuously outside of healthcare settings in the real world. This real-time, continuous flow of data can include symptoms and side effects from the patient’s perspective. Adherence to medication regimens, diet, and exercise will help build context for the efficacy of therapy.
As real-world data becomes increasingly important as a more comprehensive approach to understanding cardiovascular disease and therapies, Veradigm will continue to collect and supply high-quality data for ongoing cardiology research. Contact Veradigm for more information about using RWD for your cardiology research.
References:
- Weinberg A, Bonafede M, Reifsnyder C. Real-World Data for Cardiovascular Disease Research. Veradigm.com.
- All-time Registry counts.
- Dang A. Real-World Evidence: A Primer. Pharmaceutical Medicine. 2023;37(1):25-36. doi:https://doi.org/10.1007/s40290-022-00456-6
- Marsolo KA, Richesson R, Hammond WE, Smerek M, Curtis L. Section 2: Common Real-World Data Sources. National Institutes of Health (NIH). Updated October 14, 2022. Accessed March 28, 2023, https://rethinkingclinicaltrials.org/chapters/conduct/acquiring-real-world-data/common-real-world-data-sources/.
- Liu F, Demosthenes P. Real-world data: a brief review of the methods, applications, challenges and opportunities. BMC Medical Research Methodology. November 5, 2022;22(287)doi: https://doi.org/10.1186/s12874-022-01768-6.
- Dang A. Real-World Evidence: A Primer. Pharmaceutical Medicine. 2023;37(1):25-36. doi:https://doi.org/10.1007/s40290-022-00456-6
- Kinast B, Lutz M, Schreiweis B. Telemonitoring of Real-World Health Data in Cardiology: A Systematic Review. International Journal of Environmental Research and Public Health. 2021; 18(17):9070. https://doi.org/10.3390/ijerph18179070.
- Kinast B, Lutz M, Schreiweis B. Telemonitoring of Real-World Health Data in Cardiology: A Systematic Review. International Journal of Environmental Research and Public Health. 2021; 18(17):9070. https://doi.org/10.3390/ijerph18179070.
- Weinberg A, Bonafede M, Reifsnyder C. Real-World Data for Cardiovascular Disease Research. Veradigm.com.